Our research concept
PI message In order to achieve zero emissions and put a brake on global climate change, we must break away from carbonaceous chemical processes consuming petroleum resources and realize new “manufacturing” chemistry utilizing water, air, and related natural resources with renewable energy. In fact, gaseous water, i.e. steam can be electrolyzed as well as liquid water, and moreover, the voltage needed for the electrolysis of the former is much lower than that for the later. Hence steam electrolysis can produce ‘green’ hydrogen while minimizing the consumption of electricity generated from natural energy. In the Electronic Materials Chemistry Laboratory, we have developed a “steam electrolysis cell” that efficiently produces hydrogen from steam using electric power from renewable energy with waste heat. We are also working on the development of a “co-electrolytic cell” that produces useful compounds such as CO and NH3 by electrochemically reacting the green hydrogen with CO2 and N2 gases. The potential of the renewable energy power generation in Japan is speculated to be equivalent to twice the current total power generation in Japan. This situation indicates the energy chemists have a great opportunity to make an innovation and lead the world’s GX.
- Design of H+/H– ion conductors
- Hydride ion (H–) conducting electrodes for N2 and CO2 reduction
- Protonic solid oxide cells for steam and methane electrolysis
- プロトン(H+)/ヒドリドイオン(H–)ーヘテロイオニックセルによる水素キャリア合成・CO2再資源化
- Proton (H+) pumping effect in hydrogen permeable metal-support fuel cells
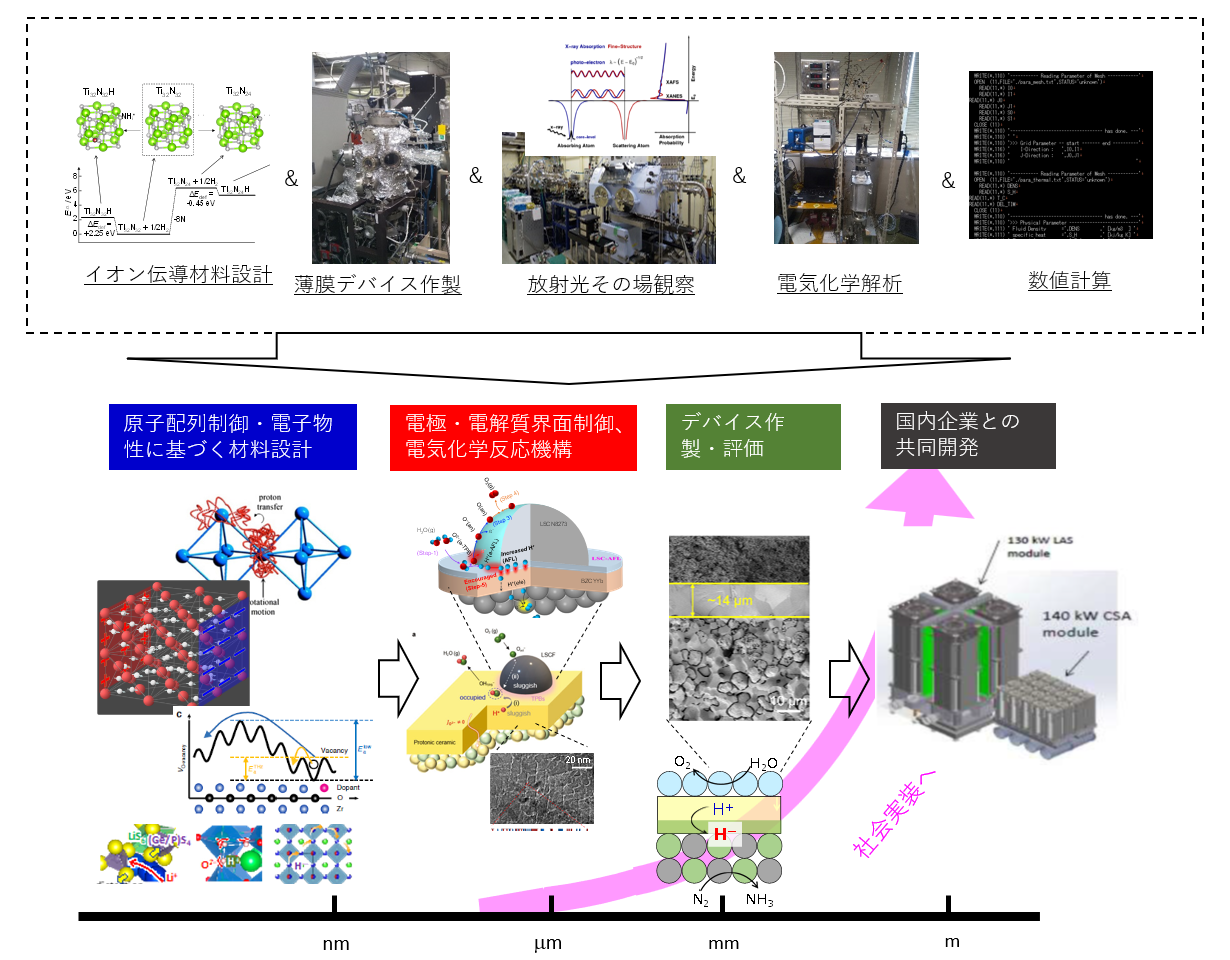
研究紹介:In Electronic Materials Laboratory, we have designed an inorganic material that generate mobile “protons (H+)” and “hydride ions (H–)” in the crystal lattices, and have developed unique inorganic ionic conductors in which hydrogen redox between H+ and H– proceeds according to the hydrogen chemical potential. Based on these materials, we also create a “solid-state ionic device” that simultaneously conducts electrochemical water splitting and reduction/hydrogenation reaction with electricity derived from renewable energy so as to produce useful chemical compounds from common raw substances such as water and air. Our skills are as follows: inorganic material design using first-principles calculation, precise structural and electronic state analysis using advanced X-ray and neutron beams, device fabrication by solid-state and vacuum processes, and electrochemical evaluations of the device performances.
Design of H+/H– ion conductors
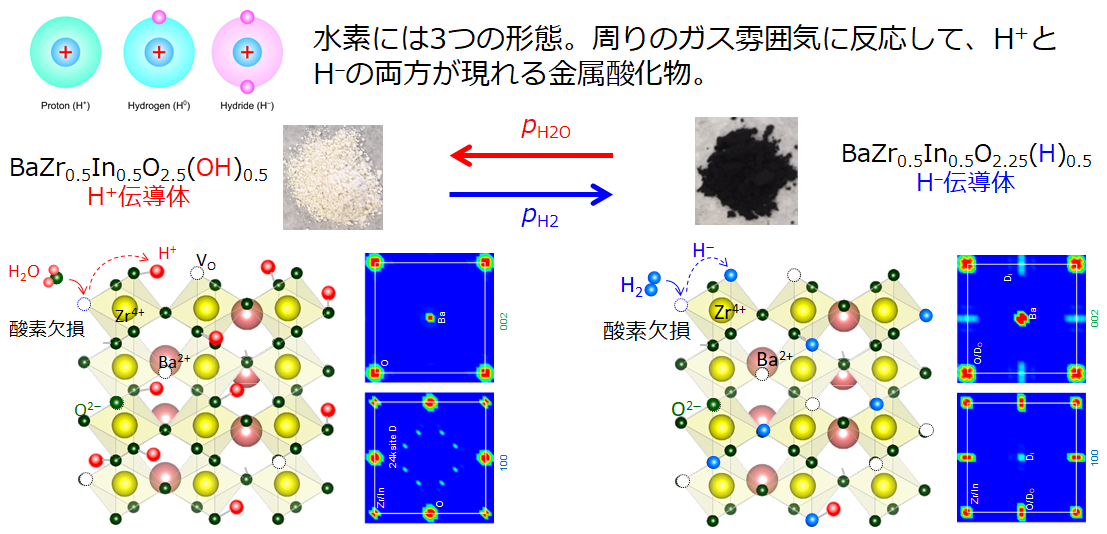
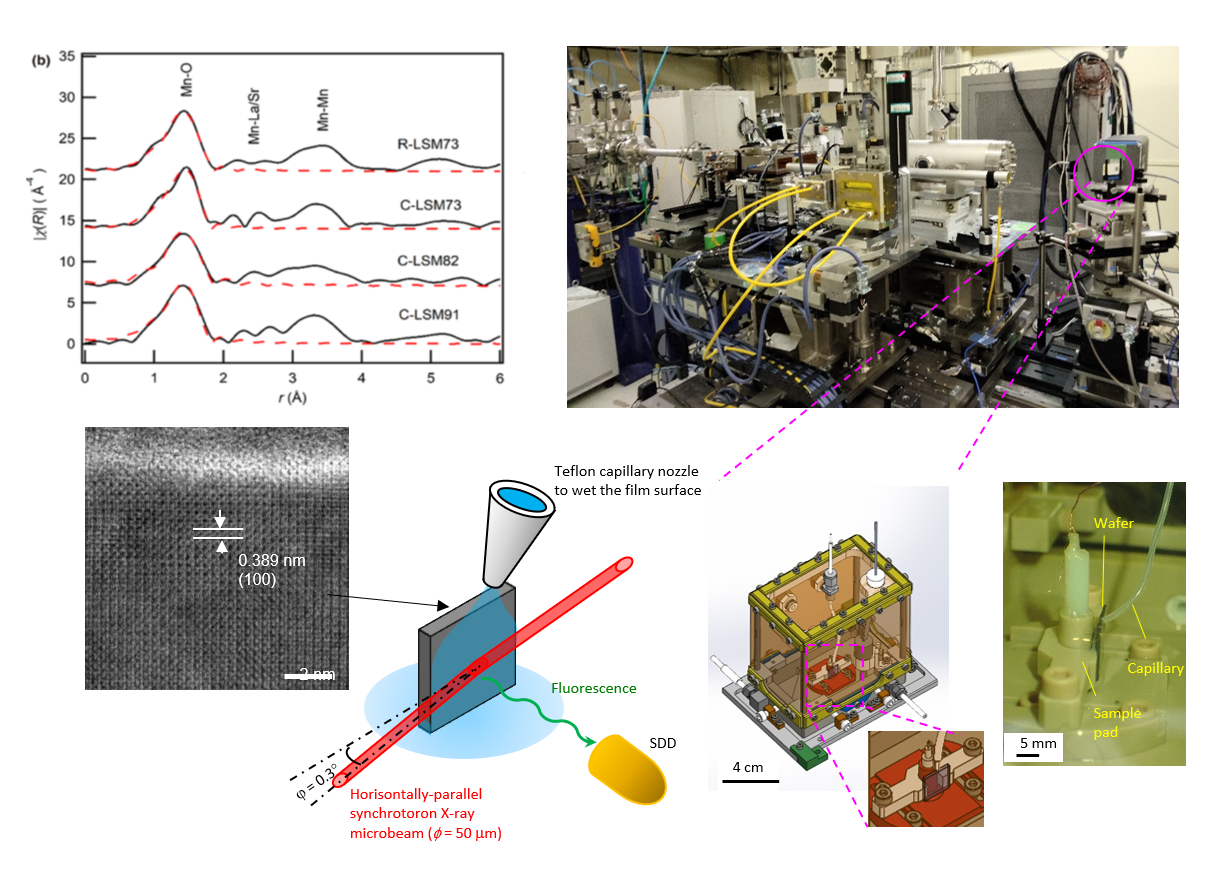
Hydrogen exists as a proton (H+) in nature, but some inorganic compounds contain its pair state, the hydride ion (H–). P-type semiconductors, especially metal oxides that generate holes in the O2p orbital, favorably form H+ carriers via the equilibria of the reactions O2p ‘hole’ and H2 gas. On the other hand, metal oxides, which are n-type semiconductors with a high electron potential (εf < -4.1 eV vs V.L.), favorably form H– carriers in the crystal. We aim , furthermore, to develop the materials that conduct H+/H– ions by designing structures that allows reduced bottleneck. For this, the materials are synthesized by solid-phase reactions. The detail structures are investigated by means of EXAFS using synchrotron radiation (Spring8, etc.) and neutron diffraction at J-Parc so as to determine the ‘position’ and ‘population’ of hydrogen atoms. Our electrochemical techniques clatify “how many“ and “how fast” H+/H- ions be mobiles in the lattice.
Hydride ion (H–) conducting electrodes for N2 and CO2 reduction
Metal oxyhydrides, which are mixed anion compounds of oxide ions (O2-) and hydride ions (H–), have recently attracted attention as various hydrogenation catalysts. Since the redox potential of H2/H– is very low, -2.3 V vs NHE, the materials that conducts H– ions are promising as a cathode of co-electrolytic cells for electrochemical ammonia synthesis and CO2 conversion. Unfortunately, many metal oxyhydrides are unstable in ambient atmosphere, and their synthesis method requires a harsh preparation condition with reducing agent such as CaH2. We demonstrated that metal nitrides MN (M=Ti, Hf, V, Nb, etc.) with small work functions favorably generate H– ion at grain boundaries simply by heating in hydrogen atmosphere, thereby exhibiting the grain boundary conduction of the H– ions . MN has been known to a thermodynamically stable and has already been industrially applied for mechanical coatings and semiconductor electronics. We aims to utilize these materials for the cathode of solid oxide electrolysis cells toward electrochemical CO2 and N2 reduction. We are conducting various electrochemical analyzes and operando analyzes using synchrotron radiation.
Protonic solid oxide cells for steam and methane electrolysis
Electrolysis of water using electricity derived from renewable energy can produce ‘green’ hydrogen without CO2 emissions. A minimum voltage of 1.5 V is required to split liquid water into O2 and H2 gases, but steam can be decomposed at a voltage of less than 1.3 V.
Methane is abundantly available from biomass resources, whereas the application is limited to green fuel owing to its low reactivity. Meanwhile, it is amazing chemistry if such methane can be upgraded to industrially-valuable organic products with the resource of renewable energy. Based on the above, we aims to develop an electrolytic cell using proton conductive Ba(Zr, Ce, Y)O3 and pyrophosphate as a solid electrolyte for electrochemical steam electrolysis and electrochemical methane coupling.

プロトン(H+)/ヒドリドイオン(H–)ーヘテロイオニックセルによる水素キャリア合成・CO2再資源化
地球規模での脱炭素化の流れを受け、化学産業も大きな転換期を迎えており、太陽光や風力を利用して化学物質や燃料を生産できる再生可能エネルギー技術への転換が強く求められています。 アンモニアは食糧生産に欠かせない化学物質ですが、その工業生産プロセスではエネルギーを大量に消費し、世界の天然ガス生産量の 3~5% を消費すると言われています。アノードでの水の酸化とカソードでの N2→NH3還元を組み合わせたアンモニア電解合成は、大量のCO2排出がなく、自然エネルギー由来の電力を使って‘グリーン’ アンモニアを合成するプロセスとして期待されています。
式
アンモニア電解合成の難しい点は、アノードにおいて、水という非常に安定な物質を酸化分解し、一方カソードでは窒素という非常に安定な気体を還元しなければならない点です。これに対しプロトンおよびヒドリドイオン伝導体を接合させた「ヘテロイオニック接合セル」が得られれば、「プロトン伝導体による水からのプロトン引き抜き・酸素発生反応促進」、および「ヒドリドイオンの強い還元力を活用した窒素還元促進」を両立することができ、効率の良いグリーン電解法になると期待されます。
Proton (H+) pumping effect in hydrogen permeable metal-support fuel cells
Protonic solid oxide fuel cells (H+-SOFCs) uses BaZr1-x-yCexMyO3-δ (M=Y, Yb, Sc, etc.; BZCM) as an electrolyte. H+-SOFCs normally take a “porous cermet-support structure“, in which a dense electrolyte thin film are formed over the porous cermet supports and a porous cathode layer are deposited on the film (a). However, the corresponding cells have not achieved the significantly high power-output performance. In contrast, fuel cells with a new structure, commonly called Hydrogen-permeable Metal-support Fuel Cells (HMFC), have been found to exhibit exceptionally high performance. This fuel cell belongs to a type of H+-SOFCs using BZCM electrolyte, but has a different cell structure, in which a hydrogen-permeable metal foil such as Pd is used as a solid anode substrate instead of the porous cermet anode (b). In the HMFC, H atoms dissolved in the hydrogen-permeable Pd alloy foil diffuse to the BZCM/Pd-oxide/metal hetero interface, and finally dissociate to protons and electrons, leading to an anodic reaction. We have found that the proton (H+) pumping effect occurs characteristically at the electrolyte/metal-interface and thus the cathode polarization and ohmic resistances are significantly decreased due to the very high water potentials at the heterointerfaces, resulting in the high output of HMFCs.


